- Atoms Of The Same Element May Contain
- Atoms Of The Same Element With Varying Number Of Neutrons
- Atoms Of The Same Element Can Have Different
- Atoms And Elements Quizlet
- Atoms Of The Same Element That Have A Different Number Of Neutrons
12) Atoms of the same element with different mass numbers are called A) ions B) neutrons C) allotropes D) chemical families E) isotopes. Answer ( 13) The current model of the atom in which essentially all of an atom's mass is contained in a very small nucleus, whereas most of an atom's volume is due to the space in which the atom's electrons move was established by A) Dalton's Atomic Theory C. The element of an atom is decided by the number of protons (which is the same as the number of electrons) in the atom. The number of protons in the atom of each element is unique, i.e.
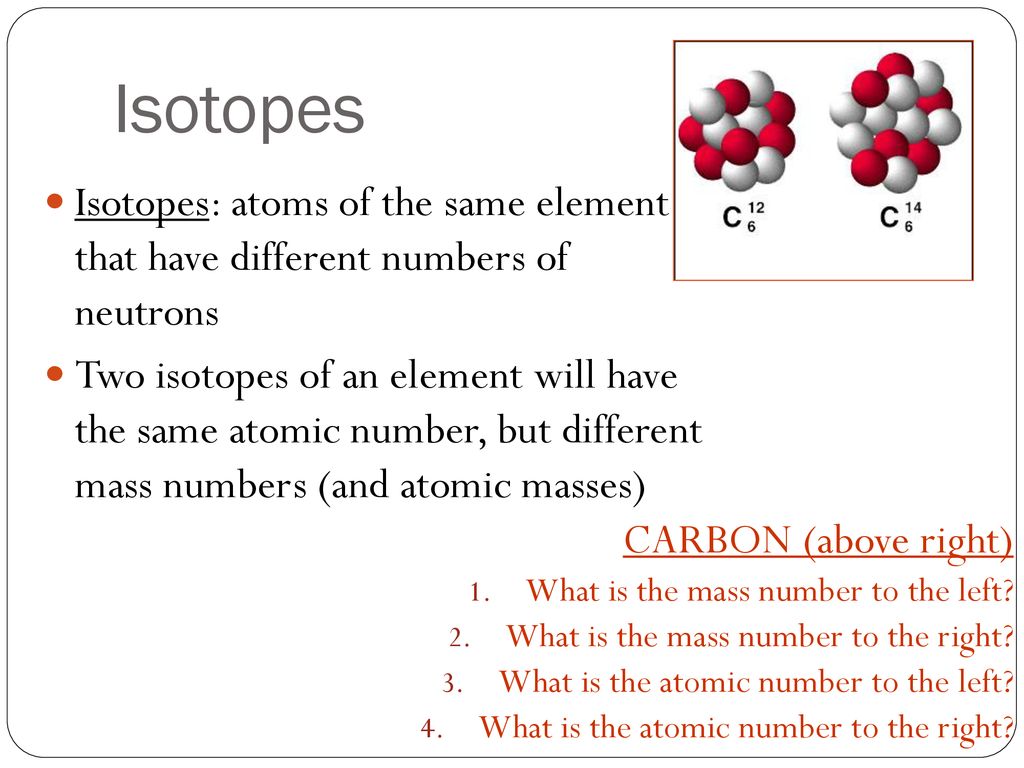
Isotopes
Isotopes are atoms of the same element that contain different numbers of neutrons. For these species, the number of electrons and protons remain constant. This difference in neutron amount affects the atomic mass (A) but not the atomic number (Z). In a chemical laboratory, isotopes of an element appear and react the same. For this reason, it is difficult to distinguish between an atom's isotopes. In contrast, nuclear scientists can identify and separate different types of atomic nuclei. The technology required for this process is more sophisticated that what could be found in a typical chemical laboratory.
The element carbon ((ce{C})) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. In a typical sample of carbon-containing material, 98.89% of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. An isotope of any element can be uniquely represented as ({}_Z^{A}X) where X is the atomic symbol of the element. The isotope of carbon that has 6 neutrons is therefore (ce{_6^{12}C}) The subscript indicating the atomic number is actually redundant because the atomic symbol already uniquely specifies Z. Consequently, it is more often written as (ce{^{12}C}), which is read as “carbon-12.” Nevertheless, the value of (Z) is commonly included in the notation for nuclear reactions because these reactions involve changes in (Z).
Most elements on the periodic table have at least two stable isotopes. For example, in addition to (ce{^{12}C}), a typical sample of carbon contains 1.11% (ce{_6^{13}C}), with 7 neutrons and 6 protons, and a trace of (ce{_6^{14}C}), with 8 neutrons and 6 protons. The nucleus of (ce{_6^{14}C}) is not stable, however, but undergoes a slow radioactive decay that is the basis of the carbon-14 dating technique used in archeology. Many elements other than carbon have more than one stable isotope; tin, for example, has 10 isotopes. There are about twenty elements that exist in only one isotopic form (sodium and fluorine are examples of these).
An important series of isotopes is found with hydrogen atoms. Most hydrogen atoms have a nucleus with only a single proton. About 1 in 10,000 hydrogen nuclei, however, also has a neutron; this particular isotope is called deuterium. An extremely rare hydrogen isotope, tritium, has 1 proton and 2 neutrons in its nucleus. Figure (PageIndex{1}) compares the three isotopes of hydrogen.
There are currently over 3,500 isotopes known for all the elements. When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. A/Z and symbol-mass formats can be used to display periodic table information. When viewing either of these two notations, isotopic differences can be obtained.

The discovery of isotopes required a minor change in Dalton’s atomic theory. Dalton thought that all atoms of the same element were exactly the same.
Look at the A/Z formats for the three isotopes of hydrogen in Table (PageIndex{1}). Note how the atomic number (bottom value) remains the same while the atomic masses (top number) are varied. All isotopes of a particular element will vary in neutrons and mass. This variance in mass will be visible in the symbol-mass format of same isotopes as well.
Atoms Of The Same Element May Contain
| Common Name | A/Z formats | symbol-mass format | Expanded Name |
|---|---|---|---|
| Hydrogen | (mathrm{^{1}_{1}H}) | (text{H-1}) | hydrogen-1 |
| Deuterium | (mathrm{^{2}_{1}H}) | (text{H-2}) | hydrogen-2 |
| Tritium | (mathrm{^{3}_{1}H}) | (text{H-3}) | hydrogen 3 |
Both A/Z or symbol-mass formats can be utilized to determine the amount of subatomic particles (protons, neutrons, and electrons) contained inside an isotope. When given either format, these mass values should be used to calculate the number of neutrons in the nucleus.
Category: Chemistry Published: March 13, 2014
Atoms Of The Same Element With Varying Number Of Neutrons
No. Two atoms of the same chemical element are typically not identical. First of all, there is a range of possible states that the electrons of an atom can occupy. Two atoms of the same element can be different if their electrons are in different states. If one copper atom has an electron in an excited state and another copper atom has all of its electrons in the ground state, then the two atoms are different. The excited copper atom will emit a bit of light when the electron relaxes back down to the ground state, and the copper atom already in the ground state will not. Since the states of the electrons in an atom are what determine the nature of the chemical bonding that the atom experiences, two atoms of the same element can react differently if they are in different states. For instance, a neutral sodium atom (say, from a chunk of sodium metal) reacts with water much more violently than an ionized sodium atom (say, from a bit of salt). Chemists know this very well. It's not enough to say what atoms are involved if you want to fully describe and predict a reaction. You have to also specify the ionization/excitation states of the electrons in the atoms. Even if left alone, an atom often does not come with an equal number of protons and electrons.
But what if two atoms of the same element both have their electrons in the same states. Then are they identical? No, they are still not identical. Two atoms of the same element and in the same electronic state could be traveling or rotating at different speeds, which affects their ability to chemically bond. Slower moving atoms (such as the atoms in solid iron) have time to form stable bonds, while faster moving atoms (such as the atoms in liquid iron) cannot form such stable bonds. A slow moving tin atom acts differently from a rapidly moving tin atom.
Atoms Of The Same Element Can Have Different
But what if two atoms of the same element both have their electrons in the same states, and the atoms are both traveling and rotating at the same speed. Then are they identical? No. Although two such atoms are essentially chemically identical (they will chemically react in the same way), they are not completely identical. There's more to the atom than the electrons. There's also the nucleus. The nucleus of an atom contains neutrons and protons bonded tightly together. The same chemical element can have a different number of neutrons and still be the same element. We refer to the atoms of the same element with different numbers of neutrons as 'isotopes'. While the particular isotope involved does not affect how an atom will react chemically, it does determine how the atom will behave in nuclear reactions. The most common nuclear reaction on earth is radioactive decay. Some isotopes decay very quickly into other elements and emit radiation, while other isotopes do not. If you are doing carbon dating, the fact that a carbon-12 atom is not identical to a carbon-14 atom is essential to the dating process. Simply counting the number of carbon atoms in a sample will not give you any information about the age of a sample. You will have to count the number of different isotopes of carbon instead.
Atoms And Elements Quizlet
But what if two atoms are the same element, have electrons in the same state, are traveling and rotating at the same speed, and have the same number of neutrons; then are they identical? No. Just like the electrons, the neutrons and protons in the nucleus can be in various excited states. In addition, the nucleus as a whole can rotate and vibrate at various speeds. Therefore, even if all else is identical, two gold atoms can have their nuclei in different excited states and behave differently in nuclear reactions.
Atoms Of The Same Element That Have A Different Number Of Neutrons
To state the case succinctly, it is very hard to have two atoms of the same element be exactly identical. In fact, succeeding in coaxing a group of atoms to be very close to identical was worthy of a Nobel Prize. With that said, don't think that atoms have individual identities beyond what has been mentioned here. If two carbon atoms are in the exact same molecular, atomic, electronic and nuclear states, then those two carbon atoms are identical, no matter where they came from or what has happened to them in the past.
